32Clinical observation of low dose esketamine for the prevention and treatment of hyperalgesia after remifentanil general anesthesia
Tao Feng, MSMS1, Gang Liu, MSMS1, Xiang-tian Yu, MSMS1, Min Li, MSMS1, Jing Tao, MSMS1, Qi-sheng, Liang PhD1
1 Department of Anesthesiology, The First Affiliated Hospital of Bengbu Medical University, Bengbu, China. *Corresponding authors at: No. 287, Changhuai Road, Bengbu, Anhui 233004, China. E-mail addresses: qishengl@qq.com (Q. Sheng).
Abstract
Background:Remifentanil is a short-acting opioid used in general anesthesia but can cause remifentanil-induced hyperalgesia (RIH). Esketamine, an NMDA receptor antagonist, is a potent anesthetic adjunct that can help prevent hyperalgesia and improve recovery outcomes. They may help improve pain management and accelerate recovery.
Objective:To investigate the effects of low-dose esketamine on the incidence of hyperalgesia, pain severity, analgesic dosage, and safety after general anesthesia with remifentanil.
Methods:Eighty patients scheduled for elective radical cervical cancer surgery were randomly assigned to either the esketamine group (Group E) or the control group (Group C), with 40 patients in each. Group E received intravenous esketamine (0.12 mg/(kg·h)) post-induction, while Group C received saline. Various parameters, including mean arterial pressure (MAP), heart rate (HR), postoperative hyperalgesia, pain onset time, VAS and OAA/S scores, analgesic pump use, and adverse reactions, were recorded.
Results:Group E had higher MAP and HR at certain time points (T3-T6) than Group C (P < 0.05). Postoperative hyperalgesia occurred in 10% of Group E patients vs. 40% in Group C (P < 0.05). Group E had a delayed first pain occurrence, lower VAS scores, and higher OAA/S scores compared to Group C (P < 0.05). Additionally, Group E used less analgesic pump solution with better postoperative analgesia (P < 0.05), with no significant difference in adverse reactions between groups (P > 0.05).
Conclusions:The application of esketamine in patients undergoing radical surgery for cervical cancer resulted in stable intraoperative hemodynamics and satisfactory postoperative analgesia and effectively prevented and controlled remifentanil-induced hyperalgesia.
Trial registration:Not applicable.
Keywords:Esketamine; Hyperalgesia; Remifentanil; Postoperative analgesia
Introduction
Remifentanil is a novel, ultra-short-acting opioid analgesic widely used in general anesthesia due to its strong analgesic effect, short action duration, fast recovery, and lack of accumulation with prolonged infusion.
However, high doses or prolonged use may lead to remifentanil-induced hyperalgesia (RIH) postoperatively, with a higher incidence in female patients, particularly in extensive procedures like radical cervical cancer surgery, which often involves high doses of intravenous remifentanil, worsening postoperative pain and reducing recovery quality (1). Given the enhanced recovery after surgery (ERAS) concept, addressing postoperative pain and preventing complications to accelerate patient recovery has become a research focus.
Studies have shown that certain sedative and analgesic interventions can effectively prevent hyperalgesia, improve patient comfort, and enhance recovery quality (2-3). Esketamine, an NMDA receptor antagonist with a stronger affinity for NMDA and opioid μ receptors than ketamine, has twice the anesthetic and analgesic potency of ketamine, making it a suitable anesthetic adjunct (4). This study examines the efficacy of low dose esketamine in preventing RIH in patients undergoing radical cervical cancer surgery.
Methods
Clinical data
Eighty patients undergoing elective radical cervical cancer surgery at The First Affiliated Hospital of Bengbu Medical University from December 2021 to September 2022 were selected. Inclusion criteria were based on the 2021 Guidelines for Cervical Cancer Diagnosis and Treatment, with pathologically confirmed cervical cancer, ASA classification II-III, BMI 18-30, and an expected surgery duration >3 hours. Exclusion criteria included neurological or psychiatric disorders, history of alcohol or drug abuse, opioid dependence, severe organ dysfunction, and drug allergies. The study was approved by the ethics committee (2021KY066), and informed consent was obtained from all patients and their families.
Anesthesia method
After monitoring was established, anesthesia was induced with midazolam, sufentanil, etomidate, and cisatracurium for tracheal intubation, followed by mechanical ventilation. Anesthesia was maintained with propofol, cisatracurium, remifentanil, and sevoflurane to maintain a BIS of 40-60. Group E received esketamine 0.12 mg/(kg·h) via IV pump, and Group C received saline. Postoperatively, patient-controlled analgesia (PCA) was provided with sufentanil, dezocine, tropisetron, and dexmedetomidine.
Observed indicators
Observed indicators included hemodynamics, postoperative hyperalgesia, pain and recovery, and adverse reactions. Hemodynamics were assessed by recording heart rate (HR) and mean arterial pressure (MAP) at T0 (pre-induction), T1 (post-induction), T2 (post-intubation), T3 (start of surgery), T4 (60 min into surgery), T5 (120 min into surgery), and T6 (end of surgery). Postoperative hyperalgesia was evaluated based on pain area expansion, persistent severe pain (VAS >8), touch/cold-induced pain, pain disproportionate to clinical findings, protective limb movements, or need for anesthesiologist intervention. Postoperative pain and recovery were measured by the time to first pain, immediate VAS and OAA/S scores, and PCA usage. Adverse reactions monitored included postoperative delirium (POD), nausea and vomiting (PONV), and skin itching.
Statistical analysis
Statistical analyses were performed using SPSS 26.0. Continuous variables were analyzed with t-tests, repeated measures with ANOVA, and categorical data with the χ² test. P<0.05 indicated statistical significance.
Results
Patients were randomized into two groups (Group E and Group C) with 40 cases each. Demographic and baseline data were comparable between groups as shown in Table 1 (P > 0.05). In this study, both groups of patients received slow induction and fluid therapy, with no use of vasoactive drugs during surgery. The HR difference between the two groups was statistically significant (F between groups = 22.286, P < 0.001), and the differences at different time points within each group were also statistically significant (F time points = 33.603, P < 0.001). There was an interaction between group and time for HR (F interaction = 4.251, P < 0.001). Compared to the C group, the HR at time points T3–T6 in the E group was significantly higher, with all differences statistically significant (P < 0.05).
Table 1. Comparison of the general condition of patients between groups
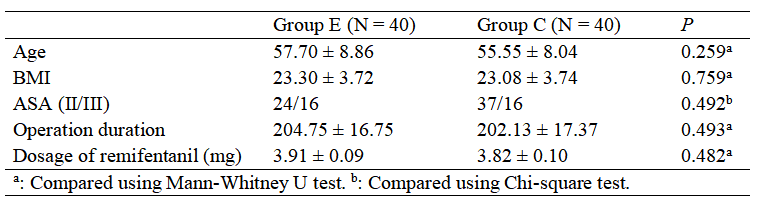
The MAP difference between the two groups was statistically significant (F between groups = 123.511, P < 0.001), and the differences at different time points within each group were statistically significant (F time points = 28.262, P < 0.001). There was an interaction between group and time for MAP (F interaction = 14.741, P < 0.001). Compared to the C group, the MAP at time points T3–T6 in the E group was significantly higher, with all differences statistically significant (P < 0.05), as shown in Tables 2.
Table 2. Comparison of surgical outcomes of patients between groups.
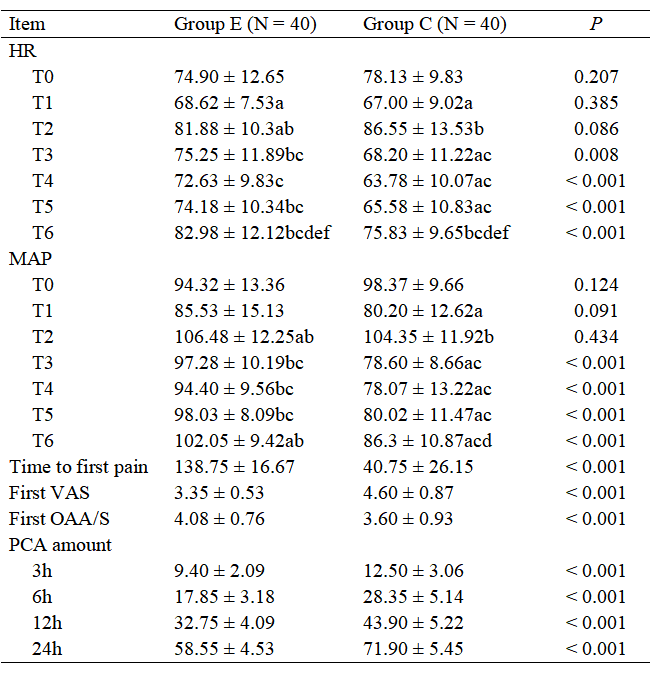
a: Compared with T0 in the same group, P < 0.05. b: Compared with T1 in the same group, P < 0.05. c: Compared with T2 in the same group, P < 0.05. d: Compared with T3 in the same group, P < 0.05. e: Compared with T4 in the same group, P < 0.05. f: Compared with T5 in the same group, P < 0.05.
The first postoperative pain time in the E group was longer than in the C group. The first pain VAS score was lower, and the first OAA/S score measured was higher in the E group (P < 0.05). The total consumption of analgesic pump medication showed an interaction between group and time (F interaction = 30.826, P < 0.001). The E group used significantly less analgesic pump medication at each time point compared to the C group, with differences being statistically significant (P < 0.05), as shown in Table 2.
According to the hyperalgesia scoring criteria, patients were assessed for hyperalgesia based on seven aspects. The occurrence of positive changes in hyperalgesia in both groups is shown in Table 3. Two or more positive indicators were considered as a positive result for hyperalgesia. The final statistics showed that in the E group, 4 cases (10%) were positive, while in the C group, 16 cases (40%) were positive. The difference between the groups was statistically significant (χ² = 9.600, P = 0.002). There was no statistically significant difference in the overall incidence of postoperative adverse reactions between the two groups (P > 0.05), as shown in Table 3.
Table 3. The occurrence of postoperative adverse events in two groups of cervical cancer patients.
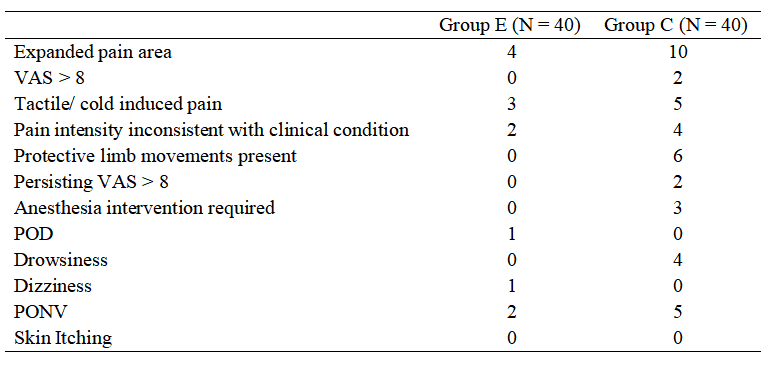
Table 6. Clinical and demographic properties of the study population
Discussion
Cervical cancer is one of the most common malignant tumors that severely threatens women's health worldwide. In 2020, China reported 109,000 new cases and 59,000 deaths from cervical cancer, accounting for 18.2% and 17.3% of the global total, respectively. Radical surgery is the preferred clinical treatment for cervical cancer. Remifentanil, a fentanyl-class μ-opioid receptor agonist, rapidly reaches blood-brain equilibrium in approximately 1 minute and is quickly hydrolyzed in tissues and blood, making its onset fast but duration short. Currently, most literature supports that the occurrence of remifentanil-induced hyperalgesia (RIH) is related to the infusion rate, duration, and dosage of remifentanil. Its prevention and treatment methods are mainly based on existing research mechanisms. Some researchers have used nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent RIH, while others have applied dexmedetomidine or the opioid hydrocodone for this purpose. Among these, the most used preventive measures are NMDA receptor antagonists or blockers.
García-Henares et al. (12) suggested that administering low-dose ketamine to patients preoperatively can effectively relieve pain and reduce postoperative opioid use. This study mainly observes the clinical effect of esketamine in preventing RIH. Esketamine is a new anesthetic and analgesic drug, the dextrorotatory form of ketamine, with greater affinity for NMDA receptors, resulting in a stronger analgesic effect. Studies by Mooney et al. (14) indicate that intravenous administration of small doses of ketamine during the perioperative period can reduce postoperative opioid use and decrease the incidence of hyperalgesia, providing some basis for the use of esketamine in preventing RIH. Esketamine has a rapid onset within 30 seconds of intravenous injection, a short half-life, mild circulatory excitation, rapid recovery, minimal respiratory depression, and few psychiatric side effects.
RIH can be classified into primary and secondary types. Primary RIH is limited to the damaged area, while secondary RIH typically manifests in areas distant from the injury site and is thought to originate from central sensitization to pain. Opioid-induced RIH is a form of secondary hyperalgesia related to diffuse injury sensitization caused by the drug. In this study, the occurrence of hyperalgesia in subjects was determined based on the presence of secondary RIH. Reports indicate that RIH onset begins 45 minutes after stopping the infusion and lasts less than 110 minutes, suggesting that, in some cases, RIH may be related only to the initial hours after surgery.
Brincke et al. (20) studied 198 patients undergoing lumbar surgery with continuous remifentanil infusion and intravenous small dose esketamine (0.12 mg/(kg·h)), and found that the pain intensity at 4 hours post-surgery was significantly reduced. Therefore, this study analyzed the effects of small dose esketamine (0.12 mg/(kg·h)) on RIH. In this study, 4 cases of hyperalgesia occurred in the E group, and 16 cases in the C group, with a statistically significant difference between the two groups.
With the introduction of the Enhanced Recovery After Surgery (ERAS) concept, anesthesia providers are now encouraged to adopt appropriate methods before and after surgery to reduce stress and complications in surgical patients, speeding up their recovery. The results of this study show that the MAP and HR of the E group were higher than those of the C group at time points T3–T6, which is mainly related to the excitatory effect of esketamine, consistent with the findings of Federera et al. (21). The total amount of analgesic used in the E group was lower than in the C group at all time points after surgery, which helps promote postoperative recovery and reduce adverse events.
In terms of postoperative OAA/S scores, the E group had higher scores, indicating better alertness and sedation levels compared to the C group. This may be related to esketamine's ability to prevent hyperalgesia, its strong analgesic effect, or its role in maintaining more stable hemodynamics during surgery. However, the exact mechanisms require further investigation. Both groups experienced varying degrees of postoperative pain, but the E group had a later onset of the first pain and a lower VAS score.
In conclusion, the use of small-dose esketamine in patients undergoing radical surgery for cervical cancer can effectively prevent and treat remifentanil-induced hyperalgesia, maintain relatively stable hemodynamics during surgery, reduce postoperative pain, and improve recovery quality.
Data sharing statement
Patient data is not available due to sensitivity reasons.
Contributors
According to the guidelines of the International Committee of Medical Journal Editors (ICMJE), all authors contributed to the four criteria. TF conceived and designed the study. GL and XTY acquired the data. ML and GL analyzed and interpreted the data. JT drafted the manuscript. TF and QSL critically revised the manuscript for valuable intellectual content. All authors read and approved the final manuscript.
Conflicts of Interest:
The authors declare that they have no competing interests.
Abbreviations
ASA: American society of anesthesiologists
BMI: body mass index
ERAS: enhanced recovery after surgery
HR: heart rate
MAP: mean arterial pressure
NMDA: n-methyl-d-aspartate
NSAIDs: nonsteroidal anti-inflammatory drugs
OAA/S: observer's assessment of alertness/sedation
PCA: patient-controlled analgesia
PONV: postoperative nausea and vomiting
POD: postoperative delirium
RIH: remifentanil-induced hyperalgesia
VAS: visual analog scale
References
- Taylor S., Noor N., Uritsi, et al. Complex regional pain syndrome: a comprehensive review. Pain Ther, 2021, 10(2): 875-892.
- Huang Y., Leem M., Lin Y., et al. Postoperative drip-infusion of remifentanil reduced postoperative pain: a retrospective observational study. Int J Environ Res Public Health, 2021, 18(17): 9225. DOI: 10.3390/ijerph18179225.
- Yang D., Tian Y., Tian G. Prevention and management of remifentanil-induced postoperative hyperalgesia. J Anesth Res, 2018, 39(8): 784-788.
- Eberls K., Koersl T., Hooft J., et al. The effectiveness of a low-dose esketamine versus alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomized controlled multicenter trial. Eur J Anaesthesiol, 2020, 37(5): 394-401.
- Sun G., Ferlay J., Siegel R., et al. Global cancer statistics 2020: global cancer incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2021, 71(3): 209-249.
- Fan X., Cai H., Pan B., et al. Comparison of dexmedetomidine and remifentanil in reducing coughing during emergence from anesthesia with tracheal intubation: a meta-analysis. Front Pharmacol, 2022, 13: 993239. DOI: 10.3389/fphar.2022.993239.
- Yuan Y., Yu Y., Wang G. Research in the prevention and treatment of remifentanil-induced hyperalgesia. Mod Oncol, 2019, 27(18): 3330-3333.
- Liu L., Liang S., Pan Z., et al. Effect of small-dose remifentanil combined with dexmedetomidine on intravenous optimization during hip replacement surgery in elderly patients. Chin Gen Med, 2022, 20(4): 606-610.
- Zhai M. Small-dose ketamine combined with NSAIDs to prevent remifentanil-induced postoperative hyperalgesia. Dissertation, Shihezi: Shihezi University, 2018.
- Li Y., Liang Q., Tao J. Clinical effects of dexmedetomidine in preventing remifentanil-induced postoperative hyperalgesia. J Bengbu Med Coll, 2018, 43(3): 299-303.
- Wang P., Fan T. Study on the half-effective dose of hydromorphone for preventing remifentanil-induced hyperalgesia in neurosurgical minimally invasive procedures. Beijing Med, 2019, 41(2): 126-128, 132.
- García-Henares J., Moral-Muñoz J., Salazar A., et al. Effects of ketamine on postoperative pain after remifentanil-based anesthesia for major and minor surgery in adults: a systematic review and meta-analysis. Front Pharmacol, 2018, 9: 921.
- Lei Y., Liu H., Xia F., et al. Effects of esketamine on acute and chronic pain after thoracoscopic pulmonary surgery under general anesthesia: a multicenter-prospective, randomized, double-blind, and controlled trial. Front Med, 2021, 8(8): 693594. DOI: 10.3389/fmed.2021.693594.
- Mooney K., Kim M., Lee H., et al. Preventive effect of ketamine on post-surgical hyperalgesia induced at the body part remote from the surgical site. Minerva Anestesiol, 2018, 84(4): 481-487.
- Eberls K., Koersl T., Hooft J., et al. The effectiveness of a low-dose esketamine versus alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomized controlled multicenter trial. Eur J Anaesthesiol, 2020, 37(5): 394-401.
- Santonocito C., Noto A., Crimi C., et al. Remifentanil-induced postoperative hyperalgesia: current perspectives on mechanisms and therapeutic strategies. Local Reg Anesth, 2018, 11: 15-23.
- Mercier R., Palmisanis S., Deblasir J., et al. Low-dose buprenorphine infusion to prevent postoperative hyperalgesia in patients undergoing major lung surgery and remifentanil infusion: a double-blind, randomized, active-controlled trial. Br J Anaesth, 2017, 119(4): 792-802.
- Li Y., Liang Q. New trends in research on opioid-induced hyperalgesia. J Anesth Res, 2017, 38(6): 563-567.
- Grapes K., Kirkham K., Frauenknecht J., et al. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trials sequential analysis. Anaesthesia, 2019, 74(6): 793-800.
- Brincke M., Maisniemi K., Kankare J., et al. Analgesic effect of intraoperative intravenous ketamine in opioid-naïve patients after major lumbar fusion surgery is temporary and not dose-dependent: a randomized, double-blind, placebo-controlled clinical trial. Anesth Analg, 2021, 132(1): 69-79.
- Federa R., Rutters R., Schiller D., et al. The emergence of ketamine as a novel treatment for post-traumatic stress disorder. Adv Pharmacol, 2020, 89: 261-286.
Share this Articles