Effect of intranasal dexmedetomidine on emergence agitation during general anesthesia in elderly patients with renal insufficiency
Qian Chang, MSMS¹, Hai-long Yue, MSMS², Yan-rong Xu, MSMS², Miao-miao Han, MSMS¹, Guo-qing Hou, PhD²
1 Hemodialysis Room, Tangshan Central Hospital, Tangshan, China. 2 Department of Anesthesiology, Tangshan Central Hospital, Tangshan, China. *Corresponding authors at: No. 601-1, Changning Road, Lubei District, Tangshan City, Hebei Province, 063000, China. E-mail addresses: 446182098@qq.com (G. Hou).
Abstract
Background: Dexmedetomidine can effectively reduce emergence agitation in elderly patients with renal insufficiency undergoing general anesthesia. This can improve patient comfort and reduce the risk of postoperative complications.
Objective: To investigate the effect of intranasal dexmedetomidine on emergence agitation during general anesthesia in elderly patients with renal insufficiency
Methods: A total of 100 elderly patients with renal insufficiency undergoing gastrointestinal surgery at Tangshan Central Hospital, Hebei Province, from February 2023 to February 2024 were selected and randomly divided into two groups of 50. The observation group received intranasal dexmedetomidine 1 hour before the end of surgery, while the control group received the same volume of 0.9% sodium chloride nasal drops at the same time. Anesthesia recovery index, pain level, sedation effect, renal function, and adverse reactions were compared between the two groups.
Results: The recovery times for spontaneous breathing and anesthesia in the observation group were shorter than in the control group (both P < 0.05). The visual analogue scale (VAS) pain scores in the observation group were significantly lower than those in the control group at 6, 12, 24, and 48 hours after anesthesia recovery (all P < 0.001). Ramsay sedation scores immediately, and at 5, 30, and 60 minutes after extubation, were higher in the observation group than in the control group (all P < 0.001). The observation group showed lower levels of blood urea nitrogen, serum creatinine, 24-hour urinary albumin, and 24-hour urinary microalbumin excretion rate than the control group (all P < 0.001). The total incidence of adverse reactions in the observation group was significantly lower than in the control group (8.0% (4/50) vs. 46.0% (23/50), χ² = 18.316, P < 0.001).
Conclusions: Intranasal dexmedetomidine in elderly patients with renal insufficiency undergoing general anesthesia provides effective analgesic and sedative effects, shortens respiratory and anesthesia recovery times, reduces agitation during recovery, promotes better renal function recovery, minimizes adverse reactions, and is safe and effective.
Trial registration: Not applicable.
Keywords: Renal insufficiency; Dexmedetomidine; General anesthesia; Emergence agitation
Introduction
Elderly patients are a unique surgical anesthesia population. As age increases, renal disease is common, particularly renal insufficiency, which can severely impact cardiovascular, hematologic, and respiratory systems, resulting in progressive decline in organ function and a high incidence of emergence agitation. Agitation increases oxygen consumption, triggers sympathetic nerve excitement, and raises perioperative risk, potentially impacting postoperative outcomes. Hence, carefully selecting anesthetics for elderly patients, improving anesthesia safety, and minimizing complications are crucial. Dexmedetomidine, an α2-adrenergic receptor agonist, has sedation, analgesic, and hypnotic effects and can effectively prevent postoperative agitation, particularly in elderly patients under general anesthesia (1-3).
Methods
Subjects
From February 2023 to February 2024, 100 elderly patients with renal insufficiency undergoing gastrointestinal surgery were selected and randomized into two groups of 50. Inclusion criteria were elective gastrointestinal surgery under general anesthesia, postoperative admission to recovery, and informed consent. Exclusion criteria included poor surgical tolerance, cognitive impairment, prolonged sedative use, and coagulation dysfunction.
Interventions
All patients received combined inhalation and intravenous anesthesia. Upon entering the operating room, a peripheral intravenous line was established in the upper extremity, and the patient's blood pressure, heart rate, and pulse oximetry were monitored. For anesthesia induction, midazolam (Jiangsu Enhua Pharmaceutical Co., Ltd., batch number: TMZ23L02) 0.03-0.04 mg/kg, remifentanil (Yichang Renfu Pharmaceutical Co., Ltd., batch number: AC4010131) 4-5 μg/kg, etomidate (Zhejiang Jiuxu Pharmaceutical Co., Ltd., batch number: TXT24A30) 0.2-0.3 mg/kg, and cisatracurium (Jiangsu Hengrui Medicine Co., Ltd., batch number: 23110411) 0.2-0.3 mg/kg were rapidly administered, followed by endotracheal intubation. For maintenance of anesthesia, propofol (Xi'an Libang Pharmaceutical Co., Ltd., batch number: 24011913), remifentanil, and cisatracurium were used. In the observation group: 1 hour before the end of surgery, dexmedetomidine (Jiangsu Enhua Pharmaceutical Co., Ltd., batch number: 24010531) 1 μg/kg was administered nasally. In the control group: 1 hour before the end of surgery, an equal volume of 0.9% sodium chloride solution was administered nasally.
Observation Metrics
Recovery indicators included time to spontaneous breathing and time to anesthesia recovery. Pain intensity was assessed using a Visual Analogue Scale (VAS) at 6, 12, 24, and 48 hours post-anesthesia, with higher scores indicating more severe pain. Sedation level was evaluated using the Ramsay Sedation Scale at immediately post-extubation and at 5, 30, and 60 minutes post-extubation (4). A score of 1 indicated an awake but restless patient, while 6 indicated deep sleep with no response to shoulder shaking. Renal function was assessed by measuring post-operative blood urea nitrogen (BUN), serum creatinine, 24-hour urinary microalbumin, and 24-hour urinary albumin excretion rate. Adverse events such as nausea, vomiting, and agitation during anesthesia recovery were recorded.
Statistical Analysis
Statistical analysis was performed using SPSS 22.0. Continuous data were presented as mean ± standard deviation and compared using t-tests. Categorical data were presented as percentages and analyzed using chi-square tests. Statistical significance was set at P < 0.05.
Results
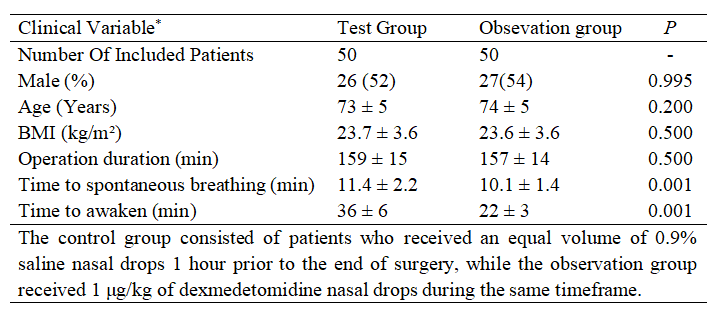
Comparison of general data and anesthesia recovery indicators between the two groups was shown in Table 1. In the observation group, there were 10 cases in stage I of renal function, 14 cases in stage II, 14 cases in stage III, and 12 cases in stage IV; in the control group, there were 14 cases in stage I of renal function, 12 cases in stage II, 12 cases in stage III, and 12 cases in stage IV. There was no statistically significant difference in the comparison of renal function stages between the two groups (P > 0.05). The time to recovery of spontaneous respiration and the time to awakening from anesthesia were both shorter in the observation group than in the control group (P < 0.05).
Patients in the observation group experienced significantly less pain after surgery, as indicated by lower VAS scores at 6, 12, 24, and 48 hours post-anesthesia compared to the control group ((2.13 ± 0.36) vs (6.54 ± 1.05), (1.04 ± 0.27) vs (4.05 ± 0.73), (0.70 ± 0.12) vs (1.05 ± 0.23), (0.54 ± 0.15) vs (1.01 ± 0.28), t=28.093, 27.346, 4.554, 6.035, all P<0.001). Additionally, the observation group demonstrated improved sedation control post-extubation, with significantly higher Ramsay sedation scores immediately after extubation and at 5, 30, and 60 minutes post-extubation ((2.35 ± 0.32) vs (1.87 ± 0.54), (2.85 ± 0.36) vs (2.28 ± 0.50), (2.70 ± 0.32) vs (2.05 ± 0.42), (2.53 ± 0.28) vs (1.80 ± 0.32). t=17.123, 6.542, 8.705, 12.140, all P<0.001).
Furthermore, the observation group exhibited better kidney function postoperatively, with significantly lower blood urea nitrogen, serum creatinine, 24-hour urine microalbumin, and 24-hour urine albumin excretion rate compared to the control group ((6.0 ± 1.0) mmol/L vs (7.8 ± 2.1) mmol/L, (105 ± 10) μmol/L vs (190 ± 15) μmol/L, (364 ± 20) mg/d vs (406 ± 25) mg/d, (1.0 ± 0.3) g/24h vs (1.9 ± 0.4) g/24h, t=32.552, 5.260, 9.057, 11.505, all P<0.001). Finally, the observation group experienced significantly fewer adverse reactions than the control group (8.0% (4/50) vs 46.0% (23/50), χ²=18.316, P<0.001).
Table 1. Comparison of baseline characteristics.
Discussion
Renal insufficiency is a common clinical kidney disease caused by severe glomerular damage, leading to disturbances in the body's metabolism, water-electrolyte balance, and acid-base balance. As the condition progresses, it can affect multiple organ systems. For example, renal insufficiency can induce cardiovascular symptoms such as hypertension, myocardial damage, arrhythmias, and heart failure, as well as severe anemia, early bleeding tendencies, and secondary respiratory diseases like bronchitis and pneumonia, and gastrointestinal symptoms such as nausea and vomiting, significantly impacting patients' quality of life and survival. With the rapid growth of the elderly population, the incidence of renal insufficiency in the elderly has increased, characterized by rapid onset, multiple complications, and high mortality rates, making timely therapeutic intervention after disease onset crucial (5).
Currently, an increasing number of elderly patients with renal insufficiency are undergoing surgery. Due to their poor underlying physical condition and the negative impact of renal insufficiency on multiple organ functions, these patients generally have a lower tolerance for surgery and anesthesia. Postoperative agitation during the recovery period is easily induced, leading to adverse events and affecting patient prognosis. Therefore, selecting safe and effective intraoperative anesthetic drugs, optimizing anesthesia plans, improving anesthesia quality, and reducing anesthetic risks are particularly crucial (6-7). In this study, nasal administration of dexmedetomidine during general anesthesia in elderly patients with renal insufficiency yielded significant results. The observation group showed significantly shorter recovery time of spontaneous respiration, anesthesia recovery time, and a lower incidence of postoperative agitation compared to the control group, laying a solid foundation for the rapid recovery of patients with renal insufficiency after surgery.
Dexmedetomidine is a novel α2-adrenergic receptor agonist that can exert sedative, hypnotic, and analgesic effects by acting on α2 receptors in the locus coeruleus. It inhibits sympathetic nerve excitation, reduces the concentration of catecholamines and norepinephrine, thereby reducing patient anxiety and delirium. It can reasonably reduce the dosage of anesthetic drugs, improve limb discomfort and sleep quality, and prevent intraoperative respiratory depression and the occurrence of postoperative agitation (8-9). In this study, the sedation effect of the observation group was significantly better than that of the control group at various stages after extubation, suggesting that dexmedetomidine has a good sedative effect. At the same time, dexmedetomidine can also bi-directionally regulate the cardiovascular system. The initial reaction is manifested as promoting peripheral vasoconstriction, which may lead to a transient increase in blood pressure, but continuous infusion will cause a gradual decrease in blood pressure, which is beneficial for eliminating sympathetic nerve tension, enhancing vagal impulses, and promoting vasodilation (10-11).
Clinical studies have shown that different doses of dexmedetomidine can increase urine output during pneumoperitoneum and the postoperative recovery period, stabilize hemodynamic fluctuations during the establishment of pneumoperitoneum, and have a certain degree of dose-dependence (12). The application of dexmedetomidine in patients undergoing laparoscopic surgery for gynecological malignant tumors can increase urine output during and after surgery, inhibit the increase of neutrophil gelatinase-associated lipocalin, and stabilize hemodynamic fluctuations during the establishment of pneumoperitoneum (12-14).
Studies have shown that the perioperative use of dexmedetomidine can significantly improve patients' cognitive function, inhibit inflammatory responses, reduce the incidence of adverse reactions, and protect renal function (15). The results of this study are consistent with this, which is of great significance for anesthesia in elderly gastrointestinal surgery patients with renal insufficiency.
In this study, the recovery of various renal function indicators in the observation group was significantly better than that of the control group, and the pain level in the 6-48 hours after anesthesia recovery was also significantly lower than that of the control group, suggesting that dexmedetomidine has good sedative and analgesic effects. After general anesthesia, the incidence of adverse reactions in the observation group was significantly lower than that of the control group, suggesting that nasal administration of dexmedetomidine in patients with renal insufficiency during surgery is safe and effective, with reduced risks and adverse reactions.
At the same time, dexmedetomidine has a good protective effect on cognitive function in elderly patients undergoing general anesthesia, and can reasonably reduce the incidence of postoperative cognitive dysfunction. Considering its mechanism of action, the use of dexmedetomidine can reduce the dosage of other anesthetic drugs and improve the accuracy of anesthesia. At the same time, dexmedetomidine can stabilize hemodynamics during surgery, prevent ischemia and hypoxia, and protect the brain system from negative stimuli, reducing the adverse effects on cognitive function, which is consistent with previous studies (16).
In conclusion, the nasal administration of dexmedetomidine for sedation and analgesia during general anesthesia in patients with renal insufficiency has good effects. It can effectively shorten the recovery time of respiration and anesthesia, reduce postoperative agitation, alleviate pain after anesthesia recovery, and improve the recovery of renal function indicators, with fewer adverse reactions and safety and efficacy. It has a good application prospect in clinical anesthesia.
Data sharing statement
Patient data is not available due to sensitivity reasons.
Contributors
According to the guidelines of the International Committee of Medical Journal Editors, all authors contributed to the four criteria. QC and GQH conceived and designed the study. QC and HLY acquired the data. YRX and MMH analyzed and interpreted the data. QC and YRX drafted the manuscript. HLY and GQH critically revised the manuscript for valuable intellectual content. All authors read and approved the final manuscript.
Conflicts of Interest:
The authors declare that they have no competing interests.
Abbreviations
BUN: blood urine nitrogen
VAS: visual analogue scale
References
- Chen J. The effects of dexmedetomidine on postoperative agitation and early cognitive function in elderly patients undergoing general anesthesia (J). Clinical Medicine, 2020, 40(1): 72-73. DOI: 10.19528/j.issn.1003-3548.2020.01.030.
- Giovannitti JA Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: A review of current clinical applications (J). Anesth Prog, 2015, 62(1): 31-39. DOI: 10.2344/0003-3006.62.1.31.
- Guo TZ, Jiang JY, Buttermann AE, et al. Dexmedetomidine injection into the locus coeruleus produces antinociception (J). Anesthesiology, 1996, 84(4): 873-881. DOI: 10.1097/00000542-199604000-00015.
- Tan SG, Lu HJ, Yin GJ, et al. Effect of low-dose dexmedetomidine nasal drop on postoperative recovery in elderly patients undergoing gastrointestinal surgery under general anesthesia (J). China Medicine, 2020, 15(5): 741-744. DOI: 10.3760/j.issn.1673-4777.2020.05.025.
- Wu YC, Huang QL, Yang ML, et al. Effect and safety of alprostadil combined with Niaoduqing granules on chronic renal insufficiency in the elderly (J). China Medicine, 2021, 16(5): 729-733. DOI: 10.3760/j.issn.1673-4777.2021.05.021.
- Tian YP. Effect and safety of dexmedetomidine in reducing agitation during anesthesia recovery period in patients undergoing neurosurgery (J). Chinese Remedies & Clinics, 2020, 20(8): 1341-1342. DOI: 10.11655/zgywylc2020.08.048.
- Zheng LH, Yang SF, Lan YP, et al. Effects of dexmedetomidine combined with hydromorphone on stress responses and restlessness during recovery from general anesthesia in elderly patients (J). Jiangsu Medical Journal, 2020, 46(3): 292-294. DOI: 10.19460/j.cnki.0253-3685.2020.03.022.
- Choi IY, Hwang L, Jin JJ, et al. Dexmedetomidine alleviates cerebral ischemia-induced short-term memory impairment by inhibiting the expression of apoptosis-related molecules in the hippocampus of gerbils (J). Exp Ther Med, 2017, 13(1): 107-116. DOI: 10.3892/etm.2016.3956.
- Colin PJ, Hannivoort LN, Eleveld DJ, et al. Dexmedetomidine pharmacodynamics in healthy volunteers: 2. Hemodynamic profile (J). Br J Anaesth, 2017, 119(2): 211-220. DOI: 10.1093/bja/aex086.
- Li JB, Lin J, Wang YQ, et al. Clinical study of dexmedetomidine combined with oxycodone on agitation during recovery from general anesthesia in elderly patients undergoing gastrointestinal surgery (J). Journal of North Pharmacy, 2022, 19(1): 105-107. DOI: 10.3969/j.issn.1672-8351.2022.01.034.
- Rao Y, Zeng R, Jiang X, et al. The effect of dexmedetomidine on emergence agitation or delirium in children after anesthesia—A systematic review and meta-analysis of clinical studies (J). Front Pediatr, 2020, 8: 329. DOI: 10.3389/fped.2020.00329.
- Xu XY, Wu W. Effect of dexmedetomidine with different dosages on the perioperative renal function in patients undergoing laparoscopic resection surgery for gynecologic malignant tumors (J). Sichuan Medical Journal, 2015, 36(11): 1549-1551, 1552. DOI: 10.16252/j.cnki.issn1004-0501-2015.11.017.
Share this Articles