Automated anesthesia systems-gastrointestinal endoscopy (AAS-GE): protocol for a multi-center, randomized controlled trial
Hai-Long Bing¹# , Shi-Ying Li¹#, Yuan-Wen Li¹, Qi-Min Wang¹, Yan Wang¹, Liu-Mei Li¹, Sheng-Qun Liu², Zhen-Hua Hu², Tian-Wei Wang³, Qing-Wang Lu⁴, Jian Huo⁵, An-Min Hu⁶, Zheng-Yuan Xia¹˒⁷, Qin-Jun Chu¹*
¹ Department of Anesthesiology and Perioperative Medicine, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou, Henan 450007, China; ² Department of Anesthesiology and Perioperative Medicine, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, Henan 450003, China; ³ Department of Anesthesiology, Anxi Hospital, Anxi, Fujian 362400, China; ⁴ Department of Anesthesiology, Jinjiang Hospital (Shanghai Sixth People's Hospital Fujian Hospital), Jinjiang, Fujian 362200, China; ⁵ Intelligent Medical Research Center, Shenzhen United Scheme Technology Company Limited, Boston, MA 02110, USA; ⁶ Shenzhen United Scheme Technology Company Limited, Shenzhen, Guangdong 518000, China; ⁷ Institute of Trauma and Metabolism, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou, Henan 450007, China; *Corresponding authors at: No. 16 Tongbai North Road, Zhongyuan District, Zhengzhou 450007, Henan, China E-mail address: jimmynetchu@163.com (Q. Chu). # These authors contributed equally.
Abstract
Introduction: Maintaining an appropriate depth of anesthesia requires precise drug titration, traditionally managed manually by anesthesiologists, which can be subject to variability and delayed responses. Recent advances in artificial intelligence (AI) have enabled the development of automated anesthesia systems that show promise in improving control accuracy, reducing drug use, and managing hemodynamic stability. With the rising demand for gastrointestinal endoscopy and a shortage of anesthesiologists, AI-assisted anesthesia offers a potential solution to enhance procedural efficiency and safety. This study protocol outlines the AAS-GE trial, which aims to evaluate the effectiveness and safety of an AI-based anesthesia system compared to conventional manual control, using ciprofol as the sedative agent.
Methods: This study proposes to conduct a 2-arm, parallel-group, multi-center, randomized controlled trial to evaluate the efficacy and safety of an AI-assisted anesthesia system (AAS-GE) compared to traditional manual anesthesia administration in patients undergoing painless gastrointestinal endoscopy, where approximately 420 patients will be included. In the control group, anesthesia will be initiated and managed by anesthesiologists based on patients’ vital signs and clinical judgment, without reference to the BIS value. In the AAS-GE Group, anesthesia was administered using AAS-GE, which delivers continuous boluses of anesthetic drugs tailored to the patient's height, weight, age, gender, and real-time monitored data, including anesthesia depth and vital signs. The primary outcome will be the incidence of hypoxemia during the procedure, defined as a SpO2 level below 92% at any point, with oxygen supplied at 3 L/min. Secondary outcomes will include hemodynamic parameters, anesthetic management and procedure metrics, and postoperative complications.
Ethics and dissemination: This protocol has been approved by the Ethics Committee of Zhengzhou Central Hospital (Approval number: ZXYY2025055. Approved 24 April 2025. Ver. 1.1). Results will be submitted to international peer- reviewed journals.
Trial registration: NCT06857344
Intelligent Anesthesia 2025;05(1):1; https://doi.org/10.63794/ia25001
Introduction
The administration of general anesthesia during surgery necessitates precise and dynamic adjustment of anesthetic agents to maintain an adequate depth of anesthesia, ensure patient safety, and facilitate rapid recovery. Traditionally, anesthesiologists manually titrate anesthetic drugs based on physiological indicators and clinical judgment, requiring continuous attention and expertise. However, this manual approach is susceptible to inter-practitioner variability and potential delays in responding to rapidly changing intraoperative conditions.
Preliminary studies have been conducted in closed-loop intravenous drug administration, demonstrating the feasibility of automated anesthesia systems even for multiple drugs (1–3). However, these systems often require additional signals such as BIS or EEG to serve as feedback (4). Recent advances in artificial intelligence (AI) and machine learning offer promising opportunities to optimize and automate anesthesia delivery. AI-based closed-loop and open-loop systems have shown potential in preclinical studies and pilot trials, improving accuracy in maintaining target anesthesia levels and reducing drug usage. Furthermore, recent evidence suggests that autonomous systems can proficiently control hemodynamic parameters, even outperforming manual control in the operating room (5,6). Despite these advancements, robust clinical evidence from well-designed randomized controlled trials remains limited.
Additionally, studies indicate that during the maintenance phase of anesthesia, the medication level established by the AI is comparable to that achieved by clinical anesthesiologists (6). Given these findings, there is growing interest in
Methods
This protocol follows the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement (11).
evaluating AI-assisted anesthesia systems in various procedural settings. One such area is gastrointestinal endoscopy, where effective sedation is crucial for patient comfort and procedural success. The demand for gastrointestinal endoscopy has been increasing due to the rising prevalence of digestive diseases and the growing emphasis on early cancer detection (7,8). However, this surge in procedures has exacerbated the shortage of anesthesiologists, highlighting the need for innovative anesthesia delivery solutions that can enhance efficiency without compromising patient safety.
The choice of anesthetic agent also plays a critical role. While propofol is widely used for sedation, ciprofol has emerged as a superior alternative for procedures such as fiberoptic bronchoscopy, gynecological procedures, gastrointestinal endoscopy, and elective surgeries. Ciprofol is associated with fewer adverse effects, particularly less painful injections, making it a preferable option (9). Meta-analysis results further support ciprofol’s higher safety profile and its ability to reduce the incidence of postoperative adverse reactions across various age groups compared to propofol (10).
The primary objective is to assess whether the AI system can sustain patients within the desired anesthetic depth range as effectively as human-controlled anesthesia by comparing drug consumptions. Secondary objectives involve evaluating the safety and practicality of the AI system, along with assessing intraoperative hemodynamic stability and the incidence of anesthesia-related adverse events. This paper presents the protocol of the AAS-GE study.
Study design
The AAS-GE (Automated anesthesia systems-gastrointestinal endoscopy) is a reinforcement learning based AI model that automatically adjusts the dose of ciprofol for automated anesthesia during painless gastrointestinal endoscopy. A 2-arm, parallel-group, multi-center, randomized controlled trial is to be carried out, aiming to prove a safety difference between AI controlled and manual anesthesia in gastrointestinal endoscopy. Figure 1 presents the flow diagram of the study, and the time schedule of the study is shown in Table 1 .
Table 1. Time schedule of the study
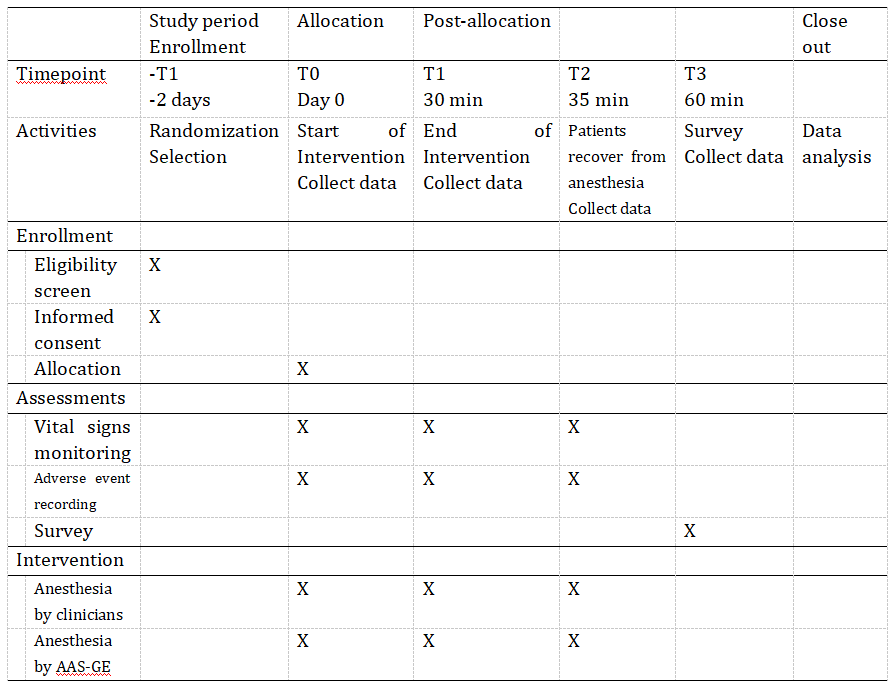
Participants
Patients undergoing elective gastrointestinal endoscopy will be assessed for eligibility. The inclusion criteria include: (1) ASA I–II classification; (2) Age between 18–65 years; (3) Expected surgery duration within 1 hour. The exclusion criteria were as follows: (1) History of heart, liver, kidney disease, diabetes, hypertension, or sleep apnea; (2) Allergy to opioids or ciprofol components; (3) Cachexia; (4) Hypothermia; (5) History of substance or alcohol abuse; (6) Breastfeeding or pregnancy; (7) Obesity: BMI > 30 kg/m²; (8) Participation in other drug clinical trials within the past 3 months; (9) Premature termination of examination due to gastric retention or inadequate bowel preparation. During recruitment, all individuals will receive information about the study objectives and the activities related to their participation and will sign an informed consent prior to their inclusion.
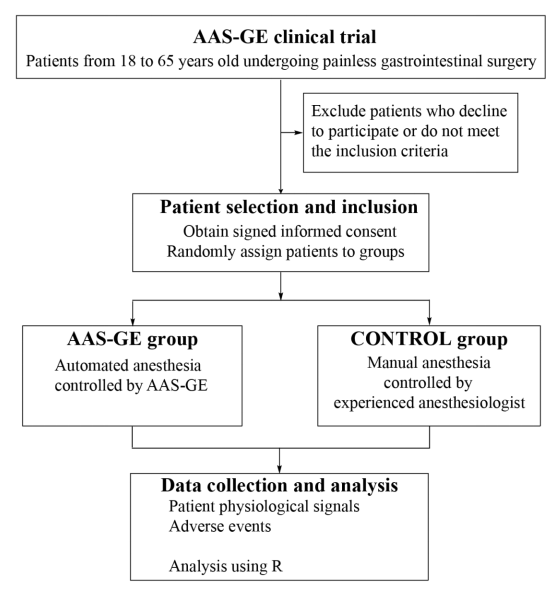
Figure 1. Study flow chart
Interventions
Anesthesiologists with over five years of clinical anesthesia experience were randomly assigned to manage the anesthesia process. They will adjust the anesthesia plan based on their clinical judgment and experience. Painless gastrointestinal endoscopy will be performed when BIS decreases to 60 or lower. At the end of procedure, a BIS score over 80 indicates that patient is fully conscious. Administration of the study drugs will be stopped when the colonoscope was removed, and all patients will be transferred to the post-anesthesia care unit (PACU). Discharge criteria are defined as a modified Aldrete score of >= 9 in the PACU post-recovery, which is assessed at 2-min intervals.
All participants will be fasted for 8 hours prior to surgery, and water intake was prohibited for 2 hours after administration of intestinal purgatives. Upon entering the endoscopy room, baseline data including physical condition, medical history, and ASA status were recorded. A 200–300 mL infusion of a balanced solution was administered via an upper dorsal vein; the physician could adjust the infusion volume based on the patient's condition.
Vital signs were recorded until each patient was fully awake. Electrocardiographic changes, heart rate, pulse oxygen saturation (SpO₂), and bispectral Index (BIS) were continuously monitored at 1-second intervals from induction to the end of the procedure. Non-invasive blood pressure was measured every 5 minutes. The average of the two consecutive systolic blood pressure (BP) will be recorded as baseline systolic BP when anesthesia starts. Oxygen was administered at 3 L/min via a nasal cannula until the patient becomes conscious after the endoscopic procedure. All gastrointestinal endoscopy procedures will be performed by experienced endoscopists. Patients were administered 0.25 μg/ml sufentanil (Renfu Pharmaceutical, Yichang, China) and 2.5 mg/ml ciprofenol (Haiske Pharmaceutical, Liaoning, China) for both induction and maintenance.
In the control group, anesthesia was initiated and managed by anesthesiologists based on patients’ vital signs and clinical judgment, without reference to the BIS value. In the AAS-GE Group, anesthesia was administered using AAS-GE, which delivers continuous boluses of anesthetic drugs tailored to the patient's height, weight, age, gender, and real-time monitored data, including anesthesia depth and vital signs.
Outcomes
The primary outcome is the incidence of hypoxemia, defined as a SpO2 level below 92% at any point from anesthesia induction to the end of examination, while receiving oxygen at 3 L/min. The secondary outcomes included: (1) The area under curve of hypoxemia; (2) The lowest SpO2 during the time interval between anesthesia induction and end of examination. (3) Anesthetic induction and maintenance dosage, where average dose is defined as the total amount of ciprofol used divided by the weight of the patient (kg) and the duration of the procedure (h); (4) the success rate of sedation, defined as achieving all preset criteria during the procedure, including maintaining oxygen saturation above 92%, no need for airway intervention, no hypotension, no need for vasoactive drugs, and no unplanned additional anesthetic drugs; (5) Awakening time: the time interval from drug administration to the first BIS lower or equal to 60; (6) Complete recovery time: the time interval the end of the examination to the first BIS greater or equal to 80; (7) Hypotension: systolic blood pressure < 90 mmHg or a 30% decrease from baseline lasting longer than 2 minutes; (8) Counts of body movements during the endoscopic procedure; (9) Doses of norepinephrine used; (10) Duration of procedure; (11) Drug-related adverse reactions,including sinus bradycardia, dizziness, and postoperative nausea and vomiting.
Allocation
The participants will be randomized using a sequence of random numbers generated by computer by an external researcher. The allocation result will be delivered through sealed envelopes.
Masking
Patients will be blinded to their allocation group. The examiners who will evaluate the outcomes during the follow-up will be blinded regarding the allocation group.
Data collection
Data collection and assessments will be made by researchers who have experience in clinical research. They will be blinded to group allocation, and they will be the same examiners at all time points for each participant to minimize interobserver variability.
Data monitoring
Since adverse events related to dental treatments are unlikely, there is no Data Monitoring Committee. However, independent oversight of the collection, management and analysis of trial data will be carried out by Qin-Jun Chu. Qin-Jun Chu, as the chief investigator, holds overall responsibility for the study and acts as the custodian of the data.
Harms
The procedures performed will follow the biosafety standards and will be performed by a trained professional. If a patient exhibited signs such as body movement, eye opening, verbal response, or other indicators of inadequate anesthesia during the procedure, additional anesthetic was administered within 10 seconds as needed. If hypoxemia (SpO2 < 92%) occurred and improvement was not achieved via the jaw-thrust maneuver, pressure support oxygen ventilation was delivered by a face mask, or tracheal intubation was necessary in case of severe hypoxemia that could not be ameliorated. When intraoperative hypotension occurs, with SBP < 90 mmHg or a MAP fall of 20 % relative to the baseline and the duration exceeding 2 min, administer intravenous norepinephrine 4–12 μg. When the HR < 50 bpm and the duration >1 min, inject 0.3–0.5 mg of atropine intravenously.
Auditing
The entered data will be audited monthly by the sponsor. Data queries will be raised as necessary, and any discrepancies identified will be promptly corrected and systematically recorded.
Data processing and analysis
Based on preliminary clinical data, the incidence of hypoxemia is expected to be approximately 20% in the conventional anesthesia group (p1 = 0.20) and approximately 10% in the AI-assisted group (p2 = 0.10). A two-sided hypothesis test will be used, with a Type I error rate (α) set at 0.05 and a statistical power (1 - β) of 0.80. Based on the sample size calculation (pooled incidence p = 0.15, with a 1:1 allocation ratio), 199 patients will be required in each group, yielding a total basic sample size of 398 participants. Considering a 5% dropout rate, a total of 420 participants (210 per group) will be enrolled to ensure sufficient valid data for analysis.
Categorical variables will be presented as frequencies and percentages, while continuous variables will be expressed as mean ± standard deviation or median (interquartile range), depending on the data distribution. The primary outcome will be compared using the χ² test, and the difference in percentages with 95% confidence intervals (CIs) will be reported. A log-binomial regression model will be constructed to adjust for age, duration of painless endoscopy, and other relevant variables, with the adjusted percentage difference and risk ratio (RR) with 95% CI reported between groups. Secondary outcomes will be compared between groups using t-tests (for symmetrically distributed continuous variables),Kruskal–Wallis tests (for asymmetrically distributed continuous variables), and χ² or Fisher’s exact tests (for categorical variables), with differences in means, medians, or percentages reported.
For variables measured at multiple time points, such as heart rate, blood pressure, and pulse oximetry, one-way repeated measures ANOVA will be used. For multiple group comparisons, continuous variables will be analyzed using ANOVA or Kruskal–Wallis tests depending on data distribution, and categorical variables will be compared using χ² or Fisher’s exact tests. Univariate logistic regression will be used to analyze factors associated with hypoxemia during general anesthesia, and odds ratios (ORs) will be reported. The 95% confidence intervals for differences in means, medians, or percentages will be calculated using the t-test, bootstrap method, and continuity-corrected Wilson method, respectively. Both risk ratios and odds ratios will be reported with 95% confidence intervals. Statistical analyses will be performed using R software (version 4.4.2). A two-sided p-value < 0.05 will be considered statistically significant.
Ethics
The study protocol has been approved by the Ethics Committee of Zhengzhou Central Hospital (Approval number: ZXYY2025055). All participants will sign an informed consent prior to enrollment. Results will be disseminated through peer-reviewed publications, presentations at major anesthesiology and gastroenterology conferences, and engagement with relevant stakeholder communities.
Discussion
The proposed randomized controlled trial is designed to rigorously evaluate the clinical performance of an AI model for autonomous anesthesia control in a real-world surgical setting. By comparing AI-driven anesthesia management to traditional manual control, this study aims to provide high-quality evidence on the potential of AI to enhance patient safety, standardize care, and support anesthesiologists in delivering optimal anesthesia. This trial also seeks to address the increasing demand for gastrointestinal endoscopy procedures and the corresponding shortage of anesthesiologists by exploring AI-driven solutions that can enhance procedural efficiency. The integration of AI-based anesthesia systems has the potential to reduce the cognitive load on clinicians, improve patient outcomes, and provide a consistent standard of care across various healthcare settings.
Hypoxemia is often defined using a threshold of 90% saturation (12). However, in this protocol, the threshold will be increased by 2% to enhance safety, as oxygen will be supplied at 3 L/min. The AUC and lowest value of SpO2, which could reflect the severity of hypoxemia, are adopted from previous studies (13,14). The findings from this trial will be instrumental in determining the safety and feasibility of AAS-GE. By assessing key parameters such as anesthetic dosage, hemodynamic stability, sedation success rate, and postoperative recovery times, this study aims to establish whether AI-driven anesthesia can reliably and effectively manage patient sedation during gastrointestinal endoscopy.
The ability of AAS-GE to maintain stable intraoperative conditions, minimize adverse reactions, and optimize drug usage will provide valuable insights into its clinical viability. If the AI system demonstrates comparable or superior performance to manual anesthesia management in terms of patient safety and procedural success, it could serve as a viable solution to address the growing demand for anesthesia services in high-volume procedural settings. Additionally, by reducing the cognitive load on anesthesiologists and standardizing sedation protocols, the implementation of AI-assisted anesthesia could contribute to more efficient resource utilization and enhanced patient care outcomes.
Ultimately, this study will help define the role of AI in modern anesthetic practice, offering evidence-based conclusions on its potential to enhance procedural safety, improve workflow efficiency, and support healthcare providers in delivering consistent and high-quality anesthesia care.
References
- Coeckelenbergh S, Joosten A, Cannesson M, Rinehart J. Closing the loop: automation in anesthesiology is coming. J Clin Monit Comput. 2024 Feb;38(1):1–4.
- West N, van Heusden K, Görges M, Brodie S, Rollinson A, Petersen CL, et al. Design and Evaluation of a Closed-Loop Anesthesia System With Robust Control and Safety System. Anesth Analg. 2018 Oct;127(4):883–94.
- Puri GD, Mathew PJ, Biswas I, Dutta A, Sood J, Gombar S, et al. A Multicenter Evaluation of a Closed-Loop Anesthesia Delivery System: A Randomized Controlled Trial. Anesth Analg. 2016 Jan;122(1):106–14.
- Xie T, Wang Y, Liu Y, Li J, Li W, Xu H. Accuracy of closed-loop and open-loop propofol delivery systems by bispectral index monitoring in breast surgery patients: a prospective randomized trial. Braz J Anesthesiol. 2024;74(2):744438.
- Zaouter C, Joosten A, Rinehart J, Struys MMRF, Hemmerling TM. Autonomous Systems in Anesthesia: Where Do We Stand in 2020? A Narrative Review. Anesth Analg. 2020 May;130(5):1120–32.
- Ren W, Chen J, Liu J, Fu Z, Yao Y, Chen X, et al. Feasibility of intelligent drug control in the maintenance phase of general anesthesia based on convolutional neural network. Heliyon. 2023 Jan;9(1):e12481.
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017 Dec 23;390(10114):2769–78.
- Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022 Mar;7(3):262–74.
- Akhtar SMM, Fareed A, Ali M, Khan MS, Ali A, Mumtaz M, et al. Efficacy and safety of Ciprofol compared with Propofol during general anesthesia induction: A systematic review and meta-analysis of randomized controlled trials (RCT). J Clin Anesth. 2024 Jun;94:111425.
- Cheng X, Zhang P, Jiang D, Fang B, Chen F. Safety and efficacy of ciprofol versus propofol for gastrointestinal endoscopy: a meta-analysis. BMC Gastroenterol. 2025 Mar 3;25(1):130.
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013 Feb 5;158(3):200–7.
- Wang X, Guo K, Sun J, Yang Y, Wu Y, Tang X, et al. Semirecumbent Positioning During Anesthesia Recovery and Postoperative Hypoxemia: A Randomized Clinical Trial. JAMA Netw Open. 2024 Jun 3;7(6):e2416797.
- Gibbs KW, Semler MW, Driver BE, Seitz KP, Stempek SB, Taylor C, et al. Noninvasive Ventilation for Preoxygenation during Emergency Intubation. N Engl J Med. 2024 Jun 20;390(23):2165–77.
- Turan A, Essber H, Saasouh W, Hovsepyan K, Makarova N, Ayad S, et al. Effect of Intravenous Acetaminophen on Postoperative Hypoxemia After Abdominal Surgery: The FACTOR Randomized Clinical Trial. JAMA. 2020 Jul 28;324(4):350–8.
Acknowledgements
This study was conducted at an academic institution, International Association for Intelligent Anesthesia. We are extremely grateful to current and future participants in the study.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article from any institutions or organizations with which they are not affiliated.
Author Contributions
All authors contributed to the conceptualization and design of the study. HLB and QJC wrote the manuscript. All authors reviewed and approved the manuscript.
Declaration of Competing Interests
The authors declare that they have no conflicts of interest.
Data Availability Statement
The data associated with the study are not publicly available but are available from the corresponding author, upon reasonable request.
Abbreviations
AAS-GE: automated anesthesia system for gastrointestinal endoscopy; AI: artificial intelligence; AUC: area under the curve; ASA: American society of anesthesiologists; BIS: bispectral index score; BMI: body mass index; BP: blood pressure; CI: confidence interval; EEG: electroencephalogram; HR: heart rate; MAP: mean arterial pressure; OR: odds ratio; PACU: post-anesthesia care unit; RR: risk ratio; SBP: systolic blood pressure; SPIRIT: standard protocol items recommendations for interventional trials; SpO₂: peripheral capillary oxygen saturation.
Rights and permissions
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
© The Author(s) 2025
Share this Articles